Authoritative Release! 41 Hospitals in Henan Province Reported, 69 Enterprises Rectified
Category:
Industry News
Time:2020-07-15
Recently, the official website of the Henan Provincial Drug Supervision and Administration Bureau published a notice on the rectification of medical device inspections in the second half of 2019, involving medical device business enterprises and user units. 41 hospitals were investigated, with ultrasound machines and respirators being the focus of the inspection.
Full text is as follows:
Notice of the Henan Provincial Drug Supervision and Administration Bureau on the Rectification of the Flight Inspection of Medical Device User Units in the Second Half of 2019

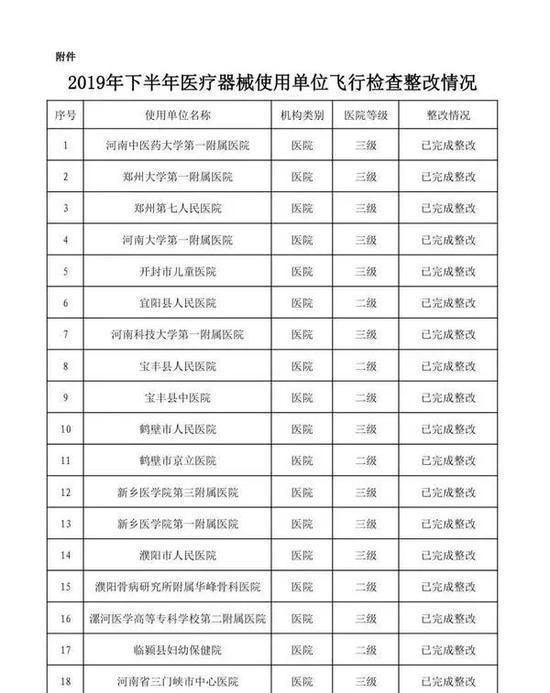
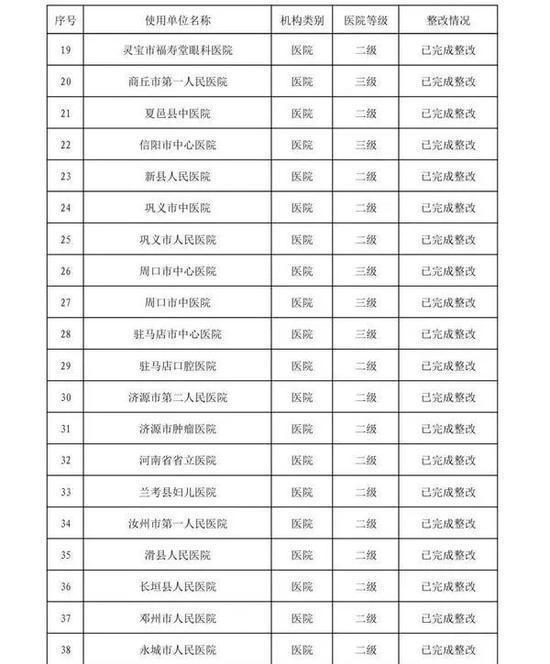
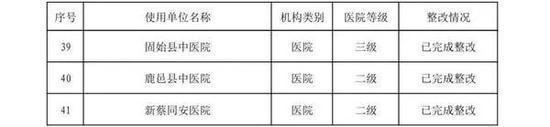
In the second half of 2019, in accordance with the "Methods for Flight Inspection of Drugs and Medical Devices", the Henan Provincial Drug Supervision and Administration Bureau organized flight inspections of 41 medical device user units.
Focusing on high-value medical consumables, sterile and implantable devices, in-vitro diagnostic reagents, sodium hyaluronate for injection, customized dentures, color ultrasound diagnostic instruments, perfluoropropane gas, infant incubators, respirators and other key products, an inspection plan was formulated. According to the "Measures for the Supervision and Management of the Quality of Medical Device Use", etc., the inspection focused on the user units' implementation of the main responsibilities for the quality and safety of medical device use and the management of the quality and safety of medical device use. A comprehensive inspection was conducted on key links such as procurement, acceptance, storage, use, maintenance, and adverse event monitoring. The inspection found that user units had varying degrees of problems such as imperfect medical device quality management systems, inadequate implementation, non-strict procurement and acceptance management, and non-standard documentation.
Regarding the problems and clues found during the inspection, the inspection team has transferred them to the regulatory departments in the location of the medical device user units for investigation and handling in accordance with relevant regulations such as the "Regulations on the Supervision and Administration of Medical Devices", the "Measures for Monitoring and Re-evaluation of Adverse Events of Medical Devices", and the "Measures for the Supervision and Management of the Quality of Medical Device Use". At the same time, user units were urged to rectify the problems.
The rectification of the flight inspection of medical device user units in the second half of 2019 is hereby announced (see attachment).
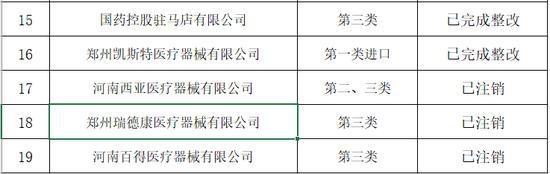
Notice of the Henan Provincial Drug Supervision and Administration Bureau on the Rectification of the Flight Inspection of Medical Device Business Enterprises in the Second Half of 2019
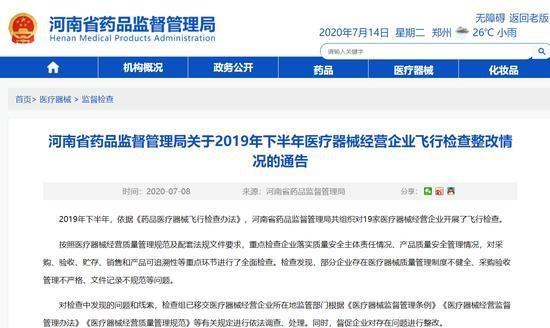
In the second half of 2019, in accordance with the "Methods for Flight Inspection of Drugs and Medical Devices", the Henan Provincial Drug Supervision and Administration Bureau organized flight inspections of 19 medical device business enterprises.
In accordance with the "Specifications for Quality Management of Medical Device Business" and supporting regulatory documents, the inspection focused on the enterprises' implementation of the main responsibilities for quality and safety, and the management of product quality and safety. A comprehensive inspection was conducted on key links such as procurement, acceptance, storage, sales, and product traceability. The inspection found that some enterprises had problems such as imperfect medical device quality management systems, non-strict procurement and acceptance management, and non-standard documentation.
Regarding the problems and clues found during the inspection, the inspection team has transferred them to the regulatory departments in the location of the medical device business enterprises for investigation and handling in accordance with relevant regulations such as the "Regulations on the Supervision and Administration of Medical Devices", the "Measures for the Supervision and Management of Medical Device Business", and the "Specifications for Quality Management of Medical Device Business". At the same time, enterprises were urged to rectify the problems.
The rectification of the flight inspection of medical device business enterprises in the second half of 2019 is hereby announced (see attachment).

Source: Henan Traffic Broadcasting 1041
Keywords:
Previous Page:

